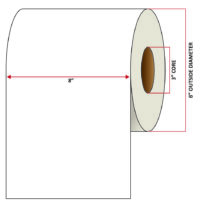
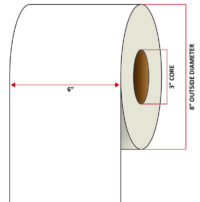
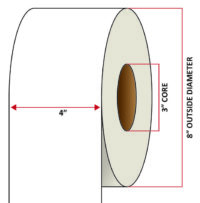
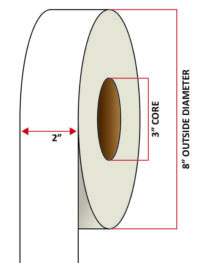
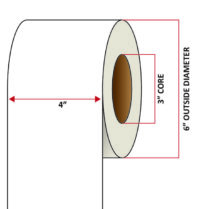
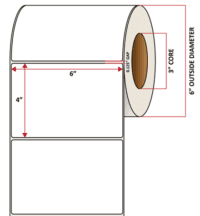
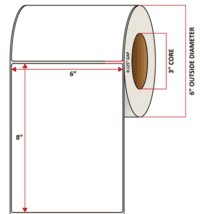
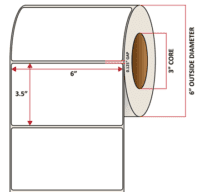
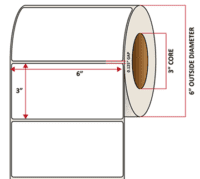
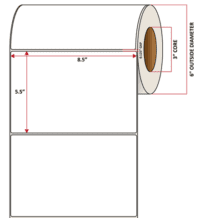
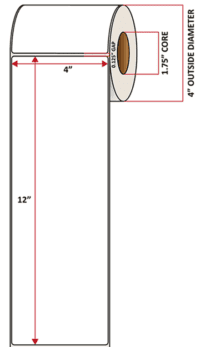
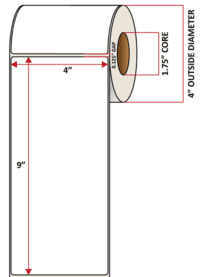
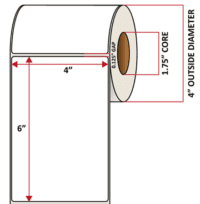
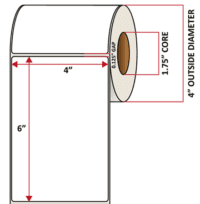
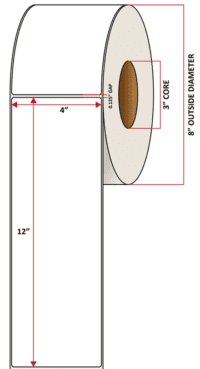
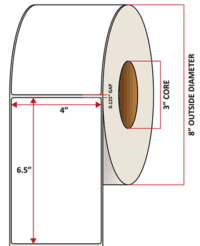
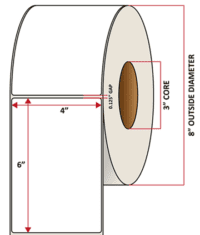
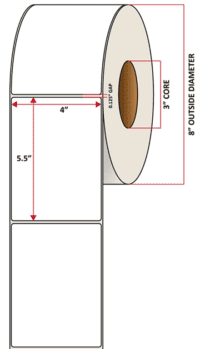
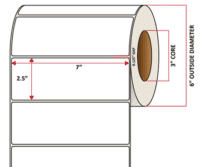
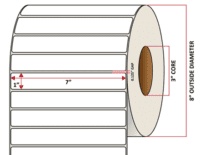
Pacific Barcode's Premium 60# Matte Coated Inkjet is an acid-free inkjet material with exceptional brightness perfect for high-resolution imaging and an excellent smear and water resistance. The tackified acrylic permanent adhesive will have good adhesion to most substrates and will withstand a service temperature of -65 to 200 degrees F with a Minimum Application Temperature of 45 degrees F.
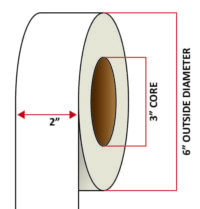
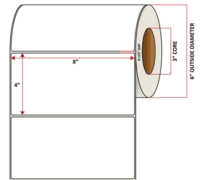
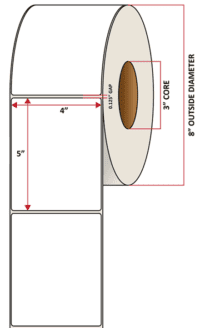
Pacific Barcode’s Premium 60# High Gloss Inkjet is a paper material which produces photo-quality prints with exceptional water resistance and high resistance to smears. The acrylic adhesive has excellent cold temperature performance and an above standard high-temperature resistance. The material has a service temperature of -65 to 200+ degrees F, and a minimum application temperature of -20 degrees F.
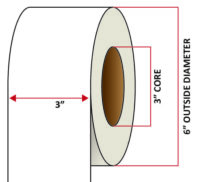
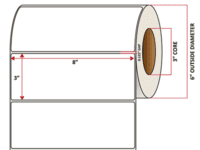
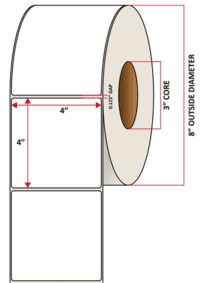
Pacific Barcode's Premium Matte Polyjet is specifically designed for inkjet-printed labels that can withstand both high and low heat while remaining stable under direct UV light; all while maintaining excellent clarity and adhesion. The acrylic permanent adhesive will retain a good adhesion on most substrates and will withstand a service temperature of -20 to 212 degrees F with a Minimum Application Temperature of 23 degrees F.
This material is especially good for labels that need to meet BS5609 requirements.
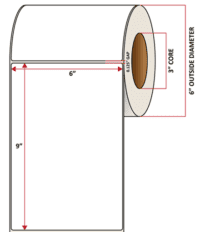
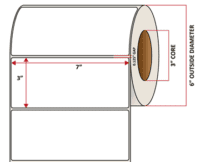
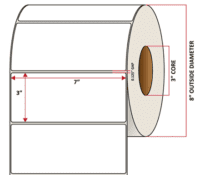
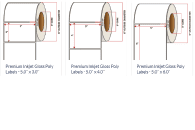
Pacific Barcode’s Premium Gloss Polyjet is specifically coated to receive dye and pigmented-based ink-jet inks and is capable of printing photorealistic images that are resistant to water and other semi-harsh chemicals. The acrylic permanent adhesive will retain a good adhesion on most substrates and will withstand a service temperature of -20 to 212 degrees F with a Minimum Application Temperature of 23 degrees F.
Pacific Barcode's 3.5 Mil Satin White BOPP is a white biaxially-oriented polypropylene film featuring good opacity and a pearl finish. It is designed for use in water-based, dye or pigment-based inkjet printers, and provides an instant-dry surface that captures brilliant colors and high-resolution images, text, and lines. The construction's small text reproducibility is excellent as well.
This material has an all-temperature adhesive is developed to provide good ambient and cold-temperature capabilities.Minimum Application Temperature:-20° F -29° C, Service Temperature Range: -65° F to +200° F
Pacific Barcode's Matte Poly (BOPP) is a white 3.5 mil biaxially-oriented polypropylene film featuring a matte finish. It is designed for use in water-based, dye or pigment-based inkjet printers, and provides an instant-dry surface that captures brilliant colors and high-resolution images, text, lines, and barcodes. It is ideal for applications that print variable information, in conjunction with full-color graphics.
This label has an all-temperature adhesive developed to provide good room temperature performance and excellent cold temperature performance. Minimum Application Temperature: -20° F -29° C. Service Temperature Range: -65° F to +200° F