Custom Medical Device Labeling & Printing Services
Medical device labeling requires a holistic approach that is essential to balance regulatory compliance, patient safety, and operational efficiency. The stakes are high, as incorrect or mislabeled medical devices can lead to grave consequences. In this context, effective branding through the use of color labels plays a pivotal role. Skillful alignment of specific colors with a brand’s identity enhances product recognition and recall, establishing a consistent and attractive presence across products. This strategy is vital not only for standing out in a competitive market but also for cementing a brand’s reputation for quality and attention to detail.
The Pacific Barcode Approach to Medical Device Labeling
- Strategic global labeling plans and content strategies.
- Lifecycle management of label components, including quality management systems.
- Multilingual safety information, patient guides, and instructions for use (IFU), including electronic versions (eIFU).
- Customization of label data and adherence to UDI compliance.
- Eco-friendly, customized labeling solutions tailored to meet regulatory demands and reduce recall risks.
Medical Device Label Examples:
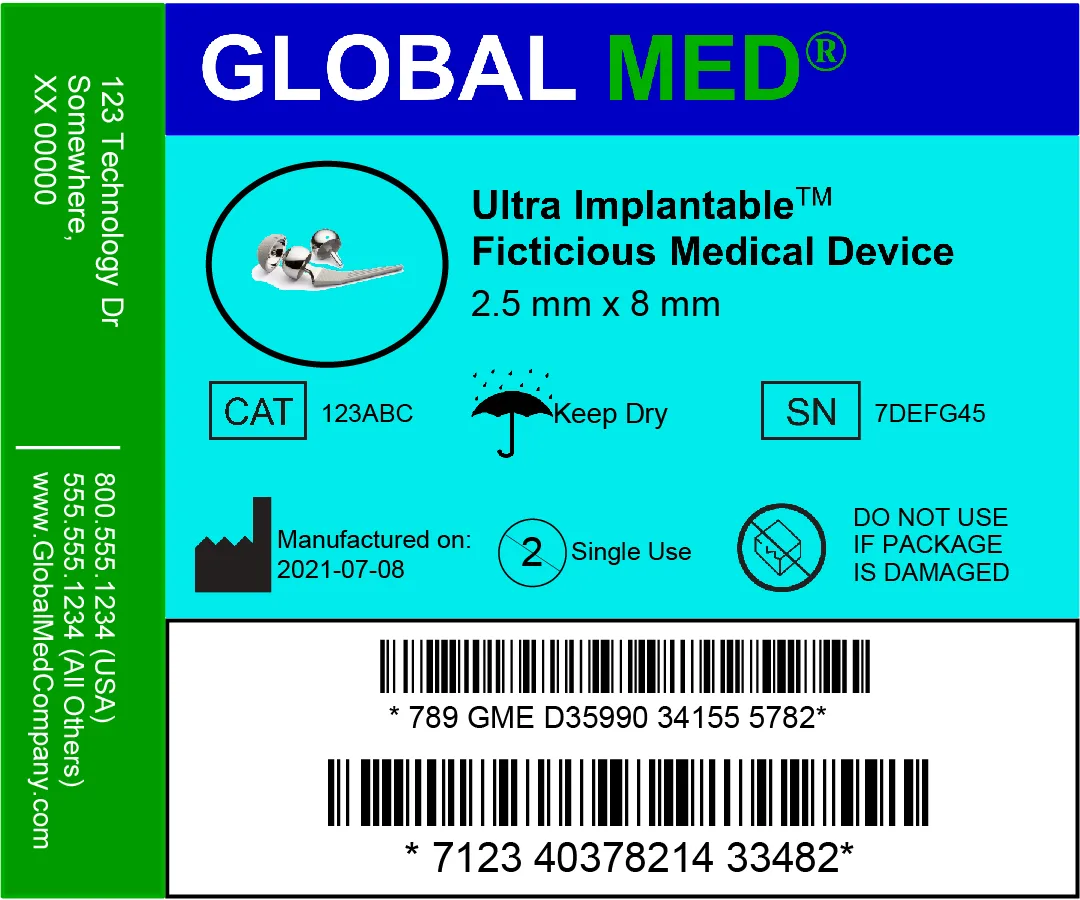

Popular Medical Device Labeling & Printing Products
Medical Device Labeling Challenges:

Regulatory Compliance: The medical device industry is subject to rigorous and often varying regulations across different countries and regions. Ensuring labels are compliant with the regulatory bodies like the FDA (U.S. Food and Drug Administration) and the EU’s Medical Device Regulation (MDR) is essential. This includes adhering to specific requirements for label content, language, symbols, and format.
Impact: Non-compliance can result in legal penalties, product recalls, and loss of market authorization. It undermines the credibility and reputation of the manufacturer.
Color: Color-coded labels can help in differentiating various important sections like warnings, usage instructions, and compliance markings, making them more noticeable and easier to understand. This can aid in better compliance with regulatory requirements.

Language and Localization: Medical devices are often distributed globally, necessitating the translation of labels into multiple languages. Ensuring accuracy in translation and localization while maintaining regulatory compliance in different markets is a significant challenge.
Impact: Misinterpretation due to poor translation or localization can lead to misuse of the device, posing risks to patient safety and potentially leading to adverse health outcomes.
Color: Color can be used to segregate information effectively in multiple languages, enhancing readability and comprehension for global distribution.

Readability and Design: Labels must be designed to ensure readability and clarity. This includes managing the size of the text and the complexity of information, especially considering the often-small size of medical devices and their packaging.
Impact: If the label is not readable or clear, it may lead to incorrect usage of the device, which can endanger patient safety and lead to medical errors.
Color: Color labels can make critical information stand out, improving the overall design and readability of medical device packaging. This is especially useful in conveying information quickly and effectively in high-stress medical environments.

Durability and Material Compatibility: Labels must be durable and resistant to various conditions such as moisture, temperature changes, and handling. The material of the label must also be compatible with the device and its sterilization processes, if applicable.
Impact: Labels that deteriorate or become unreadable compromise the device’s identification and usage instructions, increasing the risk of errors. Incompatibility with sterilization processes can also affect the device’s safety and efficacy.
Color: High-quality color labels, when used with appropriate materials, can withstand environmental challenges like moisture and temperature variations, ensuring crucial information remains legible over time.
Tracking and Traceability: With regulations like UDI (Unique Device Identification) in the U.S., there’s a growing need for effective tracking and traceability mechanisms. This often involves the integration of barcodes or RFID technology into the labeling process.
Impact: Inadequate tracking and traceability can hinder the recall process, delay the identification of defective products in the supply chain, and complicate adverse event reporting.
Color: Color coding can be integrated with barcoding and RFID technologies to enhance tracking and traceability of medical devices, aiding in swift and accurate identification.
Data Management and Version Control: Maintaining accurate and up-to-date information on labels is crucial. This requires robust data management systems and meticulous version control, especially when dealing with changes in regulatory requirements or product information.
Impact: Outdated or incorrect information due to poor data management can mislead healthcare providers and patients, leading to improper device usage or disregarded health risks.
Color: Color labels can indicate different versions or updates of a product, helping in quick identification and ensuring the correct version is used.

Risk Management: Incorrect or inadequate labeling can lead to serious risks, including misuse of the device and patient harm. Ensuring that all potential risks are clearly communicated on the label is a key responsibility.
Impact: Inadequate risk information can lead to unanticipated adverse events, compromising patient safety and potentially resulting in legal actions against the manufacturer.
Color: Visual cues like color coding can highlight risk-related information, ensuring critical warnings are not missed, thereby enhancing patient safety.
Sustainability: Increasingly, there is pressure to ensure that labeling materials and processes are environmentally sustainable, without compromising on compliance or durability.
Impact: While the direct impact on patient safety might be less immediate, unsustainable practices can harm the environment and damage the manufacturer’s public image and compliance with emerging environmental regulations.
Color: Using environmentally friendly inks and materials for color labeling can contribute to a brand’s image as responsible and sustainable.
Unique Device Identification:
Labeling and UDI (Unique Device Identification) compliance services are pivotal for medical products, offering essential information to users for enhancing safety and instructions. The sector has seen a significant rise in device recalls due to labeling errors, with a notable portion of high-risk recalls involving devices cleared under the 510k process, emphasizing the critical quality and safety challenges facing the industry.
Pacific Barcode is committed to providing comprehensive medical device labeling services, covering everything from UDI compliance to the creation and maintenance of labels throughout a product’s lifecycle. Our services streamline the entire process, from product concept to final packaging, ensuring compliance with global regulatory standards such as 21 CFR 801 and the EU’s MDR/IVDR guidelines.
Meeting Medical Device Labeling Compliance Standards
At Pacific Barcode, we offer state-of-the-art labeling equipment designed to ensure your medical devices are not only compliant with regulations such as 21 CFR 801, EU MDR, and IVDR but also meet the unique demands of your products and markets. Our equipment is engineered for precision, efficiency, and eco-friendliness, allowing you to customize labels from primary to secondary packaging, and even provide multilingual instructions for use (IFU), including electronic IFU (eIFU) options. With our technology, you can safeguard against recalls by adhering to the latest UDI compliance requirements, utilizing internationally recognized symbols, and securing the confidentiality and traceability of your data. Embrace the future of medical device labeling with Pacific Barcode’s reliable, efficient, and compliant labeling solutions.

Navigating the challenges of medical device labeling demands a comprehensive approach that carefully balances regulatory compliance, patient safety, and operational efficiency. The consequences of incorrect or mislabeled medical devices are profound, potentially leading to serious repercussions. In the context of branding, the strategic use of color labels plays a crucial role in enhancing product recognition and recall. By aligning specific colors with a brand’s identity, a consistent and appealing look across products can be achieved. This approach not only aids in differentiating the brand in a competitive market but also contributes significantly to building a reputation for quality and meticulous attention to detail.



